Tackling the cost of quality goes beyond reducing the number of defects. It involves evaluating the entire quality management system. Following are some ideas to help manufacturing organizations reduce cost while improving quality levels.
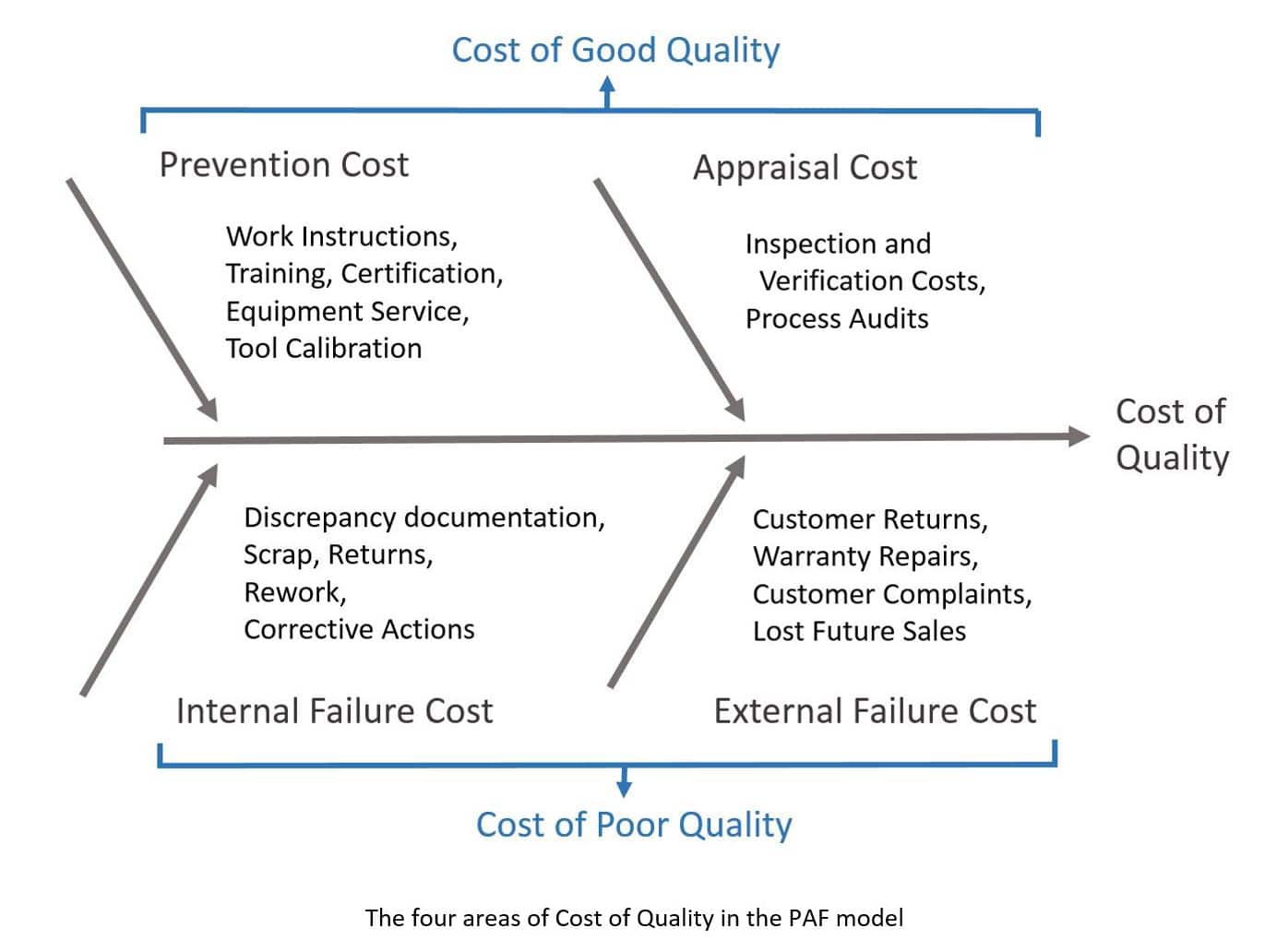
Breaking Down the Cost of Quality
The most widely accepted method for measuring and classifying quality costs is the prevention, appraisal, and failure (PAF) model which divides quality costs into the four categories in Figure 1.
The PAF model is a combination of ideas developed in the 1960’s by multiple quality gurus including Crosby and Juran. Their concepts emphasized that it is better to have more upfront investment utilizing preventive activities to minimize the costs caused by quality failures later in the product lifecycle. Research shows that the cost of poor quality (including rework, returns, and reduced repeat sales) can range from 15%-35% of business costs.
The Effect of Improving the QMS Processes on Quality Costs
There is a natural cost tradeoff between how much an organization spends on prevention versus how much it spends on fixing failures.
A traditional way to tackle reducing the cost of quality is to reduce the number of defects. This is the focus of most Six-Sigma projects. The following diagram shows how improving the sigma level would reduce defects and reduce the costs of the related failures but at some point cost of quality will go up to achieve higher levels of six sigma unless cost of quality is tackled directly.
A second way to reduce the cost of quality is to make the processes for handling preventions and failures more efficient. Figure 5 shows how the cost curves would change when the focus is on improving the quality management system itself.
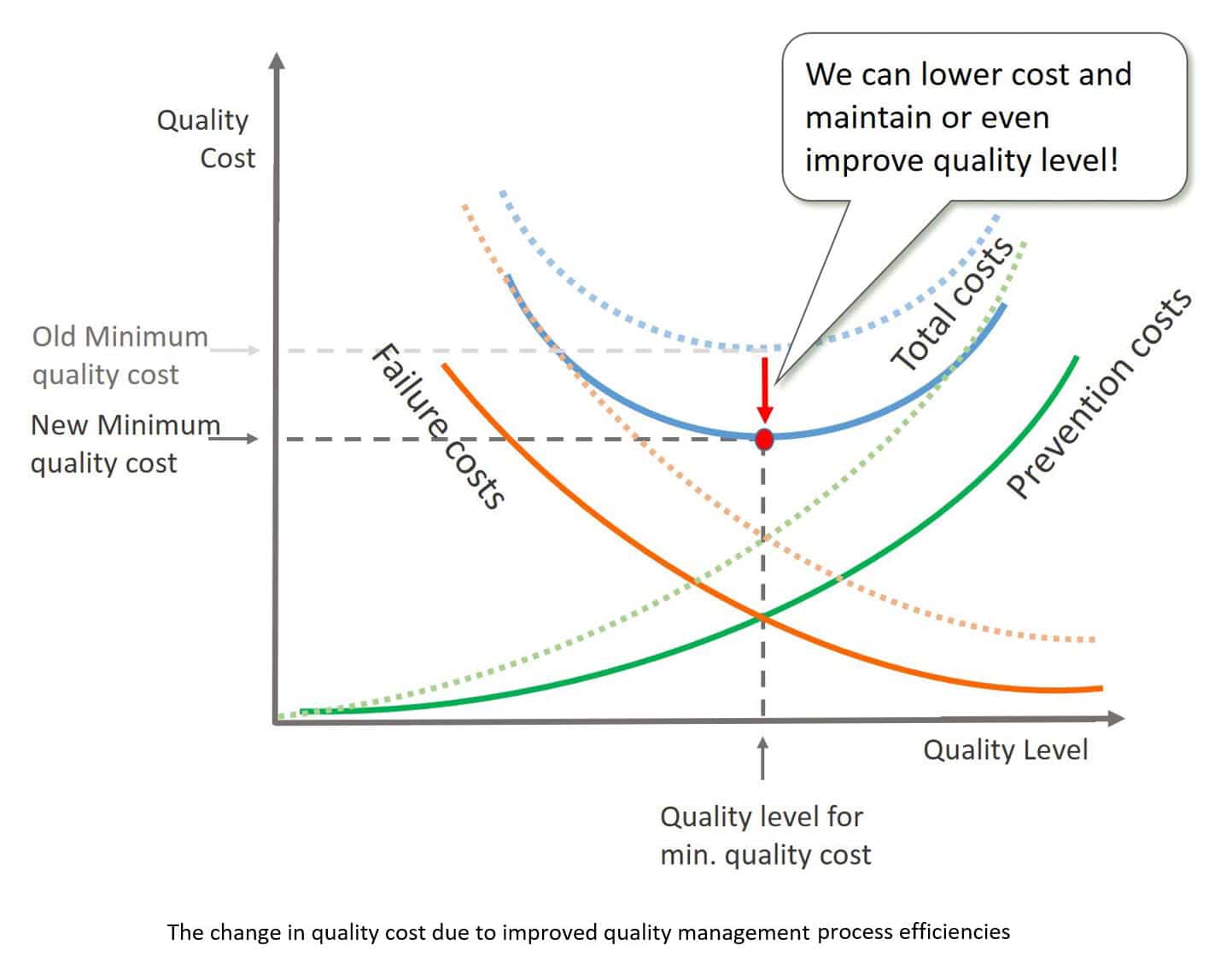
Additionally, when improving the efficiency of the quality management processes and reducing the cost of failures (cost of processing a non-conformance, cost of inspection through automation), the savings can be reinvested into better prevention methods, such as more accurate machines, better tooling, and more training, which would lead to even higher levels of quality.
The use of an MES (Manufacturing Execution System) like iBase-t’s Solumina which fully integrates an EQMS (Enterprise Quality Management System) can make a big difference to the efficiency of quality management processes. The following are some specific examples for how to reduce the cost of quality with improved quality management processes.
Reducing the Cost of Quality Failure Prevention
Prevention costs are incurred to prevent or avoid quality problems. These costs are associated with the design, implementation, and maintenance of the quality management system. They are planned and incurred before actual assembly of the product at the shop floor. The prevention costs include specifications and inspection requirements for incoming materials, processes, and finished products, worker training and gauge calibration.
#1 Work Instructions and Process Change Management
The manufacturing of a complex product like (such as an aircraft, satellite, medical device) involves the management of a continuous stream of engineering changes directed at work in process.
On a shop floor with a paper system, changes to work instructions are written into the margins with red ink and stamped by a Liaison Planner. A copy of the redlined document is routed back to a process planner to get the changes incorporated into future releases of the work instructions.
An MES can combine (1) the redlining of the work instructions on the shop floor, (2) the update of the template work instructions maintained by the Manufacturing Engineers, and (3) the notice to the shop floor operator who must acknowledge the change to the work instructions.
#2 Verifying Personnel Training and Certification
Personnel must be certified as competent based on education, training, skills, and experience. Personnel qualification processes must be standardized and documented. On paper, certification of an employee for the task is left for the supervisor to verify. An MES can validate each employee’s skills and certifications against the latest training records before they can sign on to a job. In addition, it warns the employee if the certification needs to be renewed in the next 30 days.
#3 Verifying Equipment Calibration
Equipment resources must be maintained to assure their optimal performance to capabilities, especially measurement equipment used to verify the product. The maintenance and calibration processes for equipment and tools must be standardized and documented.
An MES can verify calibration status for equipment and gauges specified to be used for data collection. Tools can be calibrated more efficiently based on actual usage instead of using the traditional date driven expiration scheme which ends up in more calibration work than is needed. Each work center is notified of tools that need to be checked in for calibration.
Reducing the Cost of Quality Appraisal
Quality appraisal activities are the most conventional quality practice and these activities have the most visible expenses. It is easy to see the cost of inspectors, testers and their equipment in the balance sheet.
Appraisal is an expensive and unreliable way of achieving quality. Appraisal in its best form is a verification that the production processes and preventive measures are working. Appraisal in its least productive form, is separating the good from the bad product, counting defects, scrapping and calculating yield. While this is a necessary part of the quality program, it is important to move towards more preventive methods to reduce the cost of failures—the cost of poor quality.
#4 Reducing Reliance on 100% Inspection
Ideally, the preference is to move away from 100% inspection and towards more inspection by production personnel; leaving only a small percentage of random over inspection for quality management personnel. An MES enables these types of strategies.
One way to reduce inspection levels from 100% inspection, 100% of the time, is to only trigger a comprehensive inspection based on production process validation (PPV) or first article inspection (FAI) rules when there is (a) a change in design, affecting fit, form or function of the product, or (b) a change of manufacturing sources, processes, inspection methods, location, tooling or materials, which affects fit, form or function.
#5 Automated Inspection
Where inspection is needed, and a lot of data needs to be collected, there might be possibility of reducing manual clerical effort using automated inspection methods such as CMM (Coordinate Measurement Machines) or visual inspection machines that are integrated directly into the MES inspection data collection records.
#6 Automated Statistical Process Control
Inspection and test results coming out of measurement and inspection machines can be imported directly into the MES. Critical measures and results can be tied to data collection points and to SPC (Statistical Process Control) run charts to monitor control levels. The automation and integration allows new levels of efficiency over the traditional processes of inspectors running spreadsheets and separate SPC software on the side.
Appraisal Costs on Supplier Parts
For assemblies manufactured internally, a company takes great care to specify detailed inspection and data collection requirements, to verify in-process the quality of a product to engineering specifications. But today, companies depend more and more on suppliers for critical subassemblies. How to ensure that the quality of subassemblies provided by suppliers are managed and given the same level of attention? Companies spend a lot of time evaluating suppliers before honoring contracts and periodically auditing them. But this type of oversight comes way short of the kind of oversight given to internally manufactured subassemblies. What if we could manage quality into the supply chain similar to how it is done internally? How can this be done efficiently with fewer personnel?
#7 Supplier Collaboration Portal
A Supplier Portal is more than a place to post files for the supplier. It is a place to collaborate online with suppliers by exchanging communications and dispatching tasks. Tasks requiring action including source inspection orders, audits, and response to discrepancies and corrective actions. Besides outstanding tasks, the portal also provides visibility to the supplier status and supplier performance ratings.
#8 Source and Receiving Inspection
Current practices for supplier quality appraisal focus on supplier certification and receiving inspection. However, companies are also minimizing inventory levels and pushing delivery dates as close as possible to the point of use. What happens when an issue is discovered during receiving inspection? Oops, instant impact on production schedule! This is why companies today are looking to move more inspection requirements into the supply chain using more source inspections. Inspection templates allow requirements to be managed for both source and receiving locations. Source inspection orders are then dispatched to suppliers through the supplier portal.
Reducing the Cost of Quality Failures
During inspections performed at production, receiving or supplier site, it is possible to find some aspects of products, materials and components to be nonconforming to specifications. These nonconformances could lead to rework, scrapping, returns, and recalls; all of which should be documented in nonconformance or discrepancy reports and classified in a database in such a way that the organization can use the data to determine costs and areas for improvement.
#9 Discrepancy Documentation
The cost of filling out a non-conformance report on manufactured or purchased parts includes the cost of good problem Identification. The use standard codes and descriptions for symptom, defect and cause types, can simplify (and speed up) the documentation process, and provide more consistent data for analysis.
#10 Workflow Driven Routing of Tasks
The costs of routing a discrepancy for disposition through a workflow process across multiple departments can be controlled by limiting the participants in each discrepancy. Limit the participants to those who are needed, instead of including the entire multi-discipline Material Review Board (MRB) in every review. By adjusting the workflow with a different escalation process for different types of issues and disposition actions, it is possible to make the best use of everyone’s time.
#11 Rework, Re-inspection, and Re-testing
One of the advantages of the integration of the EQMS and MES is the handling of rework instructions to correct an issue. The integration makes it easy to use the same process planning tools, to either append work to the work order or edit the instructions for the affected units only. This is done as part of the integrated discrepancy disposition workflow. The resulting rework instructions and operations appear in the same job list, along with the planned work, so technicians at the shop floor do not have to learn a different process for rework, re-inspection and re-testing. However, these activities do get classified (and segregated in reporting) to the financial system as cost of poor quality.
The Cost of External Quality Failures
The intangible costs of external quality failures (including customer dissatisfaction, loss of reputation and loss of future sales) might be hard to calculate, but are not hard to picture as having a huge negative impact on the future of the business. The best way to reduce external failure cost is to not have them at all! The best way to avoid external quality failure costs is to focus on improving the other three costs of quality.
Conclusion
There is a natural cost tradeoff between how much an organization spends on prevention versus how much it spends on fixing failures. In addition to the traditional way of reducing the cost of quality by reducing the number of defects, it is possible to tackle the efficiency of the quality management system itself to further reduce cost of quality.
Using an integrated MES and EQMS software solution leads to more embedded quality control throughout the entire manufacturing process, more ownership of quality by production personnel, and more smiles and fewer pains for everyone in the organization.




